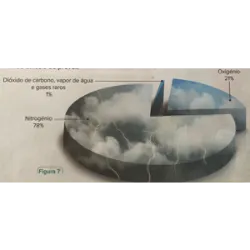
Curiosities, games, challenges and quiz on various topics

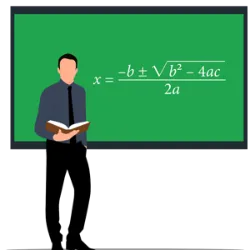
Heat is a form of energy that is transferred from one body to another when there is a difference in temperature between them, resulting in thermal equilibrium. The standard unit of measurement for heat is the Joule (J), although it is common to use calories (cal) for measurement. Heat and temperature are distinct concepts, with the first being energy and the second a measure of the agitation of particles in a body. Throughout history, the concept of heat has been debated by philosophers and scientists, from Aristotle to Lavoisier and Lord Kelvin, who established that heat is a form of energy. Heat can be transferred by conduction, convection and radiation, the latter being a propagation of electromagnetic waves that does not require a physical medium to occur, following the Steffan-Boltzmann Law, where the amount of heat emitted is proportional to the fourth power of the temperature of the body (Q α T^4).